Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake
Abstract
:1. Introduction
2. Intracellular Transport of NPs
2.1. Active Transport
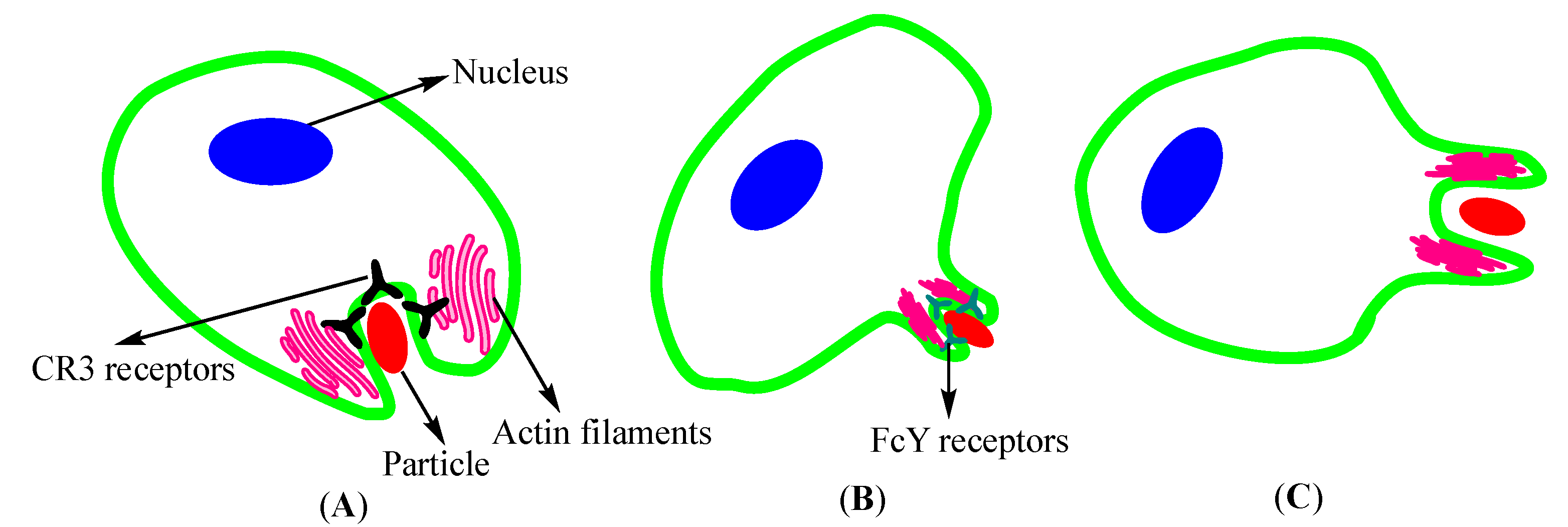
2.1.1. Endocytosis
2.1.2. Exocytosis
2.1.3. Ion Pumps
2.2. Passive Transport of NPs
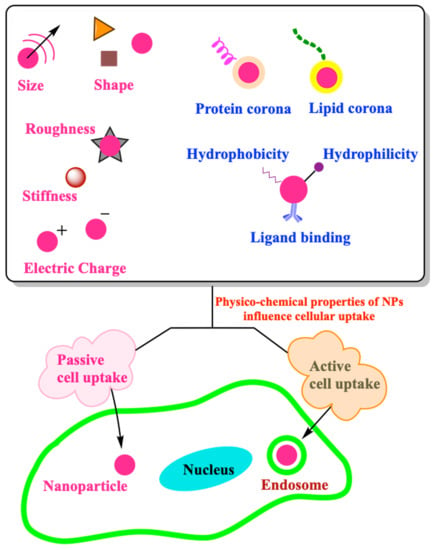
3. Role of Physico-Chemical Properties of NPs in Cell Uptake
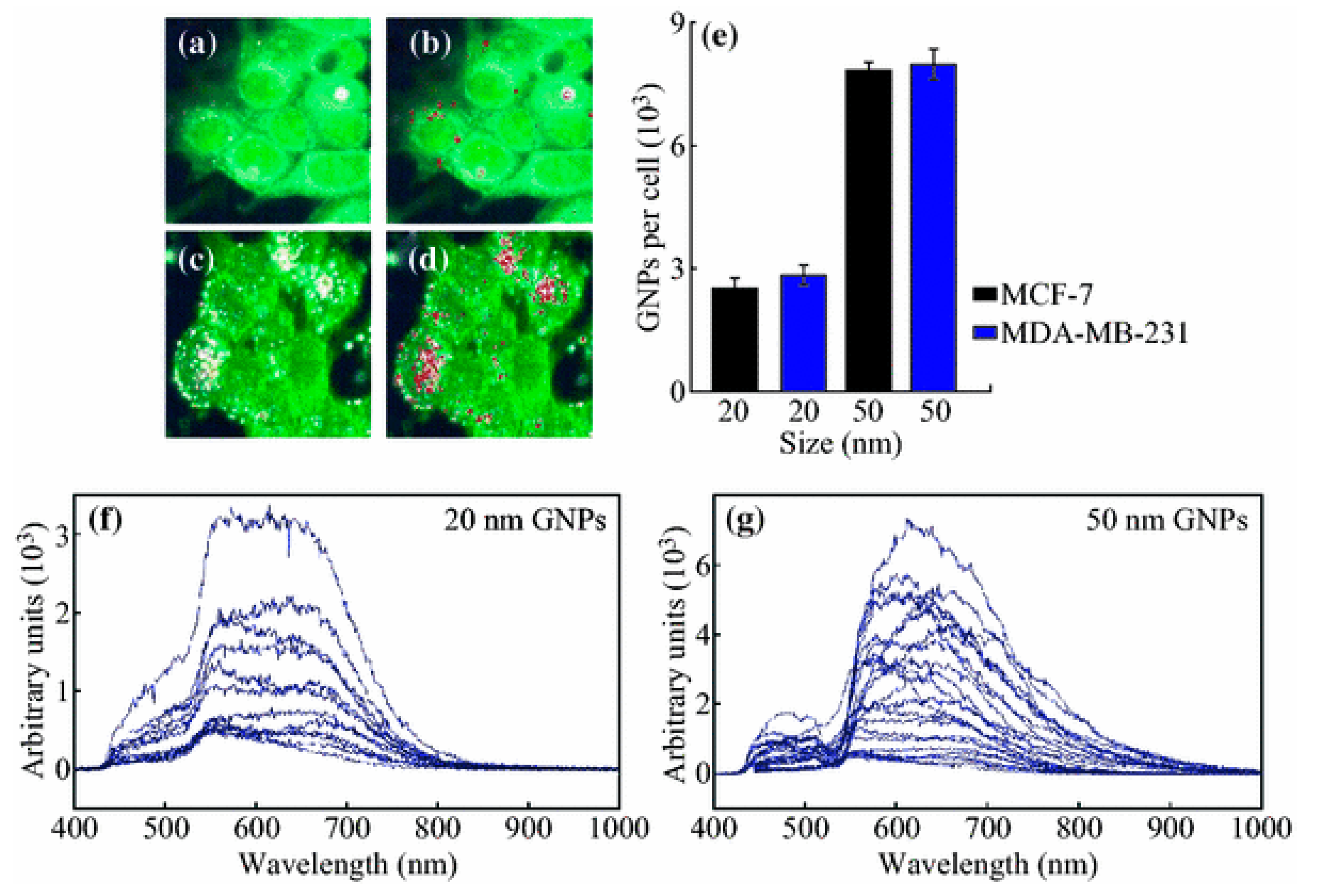
3.1. Size
3.2. Shape
3.3. Corona
3.4. Surface Chemistry
3.4.1. Surface Electrical Charge
3.4.2. Hydrophobicity and Hydrophilicity
3.4.3. Ligand Binding
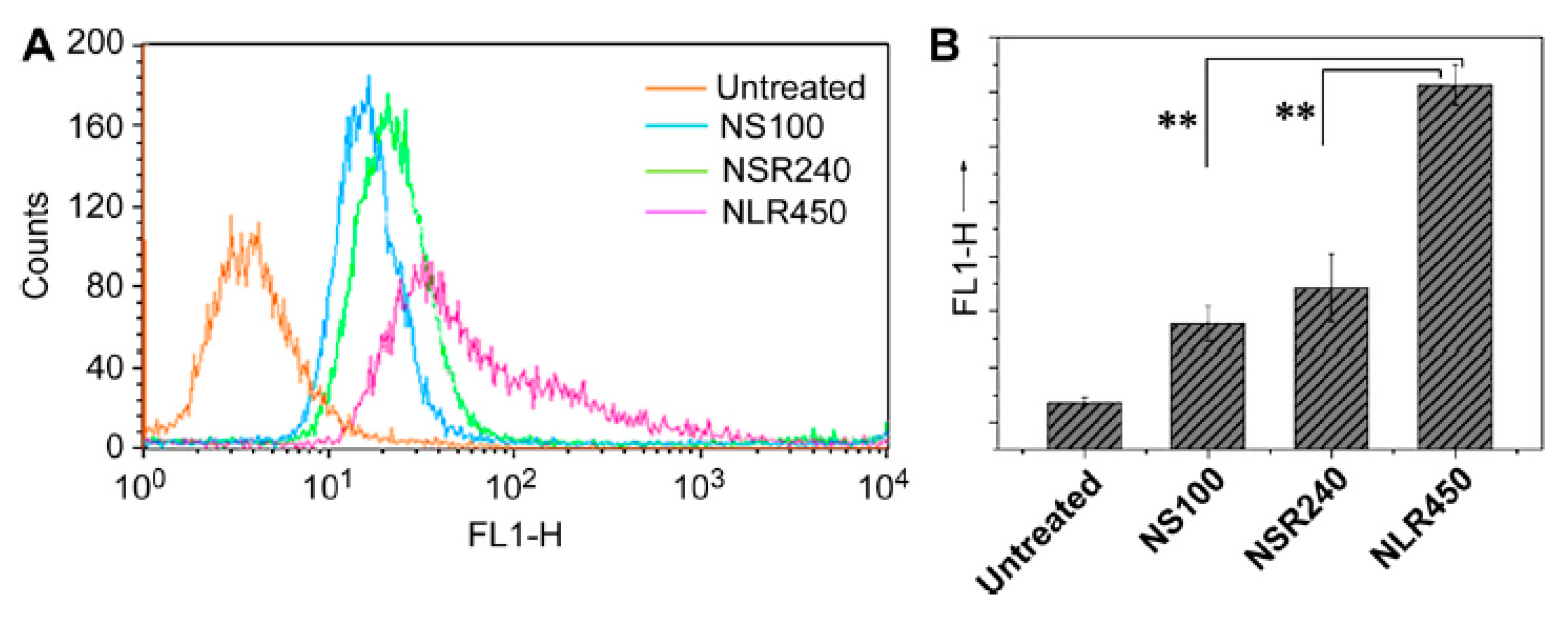
3.5. Mechanical Properties
4. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Jahromi, L.P.; Panah, F.M.; Azadi, A.; Ashrafi, H. A mechanistic investigation on methotrexate-loaded chitosan-based hydrogel nanoparticles intended for CNS drug delivery: Trojan horse effect or not? Int. J. Biol. Macromol. 2019, 125, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Mathe, V.; Dongre, P. Albumin nanoparticles conjugates binding with glycan-A strategic approach for targeted drug delivery. Int. J. Biol. Macromol. 2019, 126, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Al Zaabi, A.M.; Merheb, M.; Matar, R. Nanoparticles in Gene Therapy. Int. J. Integr. Biol. 2016, 17, 7–16. [Google Scholar]
- Kulkarni, S.A.; Feng, S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Ma, N.; Ma, C.; Li, C.; Wang, T.; Tang, Y.; Wang, H.; Mou, X.; Chen, Z.; He, N. Influence of nanoparticle shape, size, and surface functionalization on cellular uptake. J. Nanosci. Nanotechnol. 2013, 13, 6485–6498. [Google Scholar] [CrossRef]
- Rubinsky, B. Irreversible electroporation in medicine. Technol. Cancer Res. Treat. 2007, 6, 255–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.-C. Microinjection as a tool of mechanical delivery. Curr. Opin. Biotechnol. 2008, 19, 506–510. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Campagnolo, L.; Fadeel, B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small 2013, 9, 1557–1572. [Google Scholar] [CrossRef] [Green Version]
- Bode, G.H.; Coué, G.; Freese, C.; Pickl, K.E.; Sanchez-Purrà, M.; Albaiges, B.; Borrós, S.; van Winden, E.C.; Tziveleka, L.-A.; Sideratou, Z.; et al. An in vitro and in vivo study of peptide-functionalized nanoparticles for brain targeting: The importance of selective blood–brain barrier uptake. Nanomedicine 2017, 13, 1289–1300. [Google Scholar] [CrossRef]
- Backes, W.L. Passive Diffusion of Drugs across Membranes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–5. [Google Scholar]
- Stillwell, W. An Introduction to Biological Membranes: Composition, Structure and Function; Elsevier: Amsterdam, The Netherlands, 2016; p. 590. [Google Scholar]
- Gouaux, E.; MacKinnon, R. Principles of selective ion transport in channels and pumps. Science 2005, 310, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, L.; Li, L.; Zhou, Z.; Wang, F.; Xiong, X.; Zhou, R.; Huang, Y. Programmed drug delivery system based on optimized “size decrease and hydrophilicity/hydrophobicity transformation” for enhanced hepatocellular carcinoma therapy of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Kiwada, H.; Ishida, T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J. Control. Release 2013, 172, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, L.; Liang, K.; Liu, M.; Liu, X.; Song, Y.; Deng, Y. The accelerated blood clearance phenomenon of PEGylated nanoemulsion upon cross administration with nanoemulsions modified with polyglycerin. Asian J. Pharm. Sci. 2018, 13, 44–53. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Shi, G.; Ni, C. Zwitterionic pH/redox nanoparticles based on dextran as drug carriers for enhancing tumor intercellular uptake of doxorubicin. Mater. Sci. Eng. C 2016, 61, 278–285. [Google Scholar] [CrossRef]
- Chen, D.; Wang, J.; Wang, Y.; Zhang, F.; Dong, X.; Jiang, L.; Tang, Y.; Zhang, H.; Li, W. Promoting Inter-/Intra-Cellular Process of Nanomedicine through its Physicochemical Properties Optimization. Curr. Drug Metab. 2018, 19, 75–82. [Google Scholar] [CrossRef]
- Kumari, S.; Swetha, M.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovitch, M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Colucci-Guyon, E.; Niedergang, F.; Wallar, B.J.; Peng, J.; Alberts, A.S.; Chavrier, P. A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr. Biol. 2005, 15, 2007–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, J.A.; Baer, S.C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995, 5, 89–93. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Pelkmans, L.; Helenius, A. Endocytosis via caveolae. Traffic 2002, 3, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.G. The caveolae membrane system. Annu. Rev. 1998, 67, 199–225. [Google Scholar] [CrossRef] [Green Version]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E.; Liu, J.; Oh, P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J. Biol. Chem. 1995, 270, 14399–14404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J. Cell Mol. Med. 2009, 13, 1228–1237. [Google Scholar] [CrossRef] [Green Version]
- Lamaze, C.; Dujeancourt, A.; Baba, T.; Lo, C.G.; Benmerah, A.; Dautry-Varsat, A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol. Cell. 2001, 7, 661–671. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Almers, W. Exocytosis. Annu. Rev. Physiol. 1990, 52, 607–624. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.-B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201, 18–29. [Google Scholar] [CrossRef]
- Berg, J.; Tymoczko, J.; Stryer, L. Biochemistry; W. H. Freeman and Company: New York, NY, USA, 2002. [Google Scholar]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; p. 1168. [Google Scholar]
- Cooper, G.M.; Hausman, R. A Molecular Approach: The Cell; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Haque, S.; Pouton, C.W.; McIntosh, M.P.; Ascher, D.B.; Keizer, D.W.; Whittaker, M.; Kaminskas, L.M. The impact of size and charge on the pulmonary pharmacokinetics and immunological response of the lungs to PLGA nanoparticles after intratracheal administration to rats. Nanomedicine 2020, 30, 102291. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Feliciano, T.J.; Li, W.; Lee, A.; Odom, T.W. Gold nanoparticle size and shape effects on cellular uptake and intracellular distribution of siRNA nanoconstructs. Bioconjug. Chem. 2017, 28, 1791–1800. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-J.; Wang, J.-L.; Iqbal, S.; Li, H.-J.; Cap, Z.-T.; Wang, Y.-C.; Du, J.-Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorff, T.; Gruber, A.; Eravci, M.; Dumbani, M.; Klinger, D.; Haase, A. Amphiphilic nanogels: Influence of surface hydrophobicity and cellular uptake. Int. J. Nanomed. 2019, 14, 7861–7878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, C.; Jana, N.R. Galactose multivalency effect on the cell uptake mechanism of bioconjugated nanoparticles. J. Phys. Chem. C 2018, 122, 25651–25660. [Google Scholar] [CrossRef]
- Eshaghi, B.; Alsharif, N.; An, X.; Akiyama, H.; Brown, K.A.; Gummuluru, S.; Reinhard, B.M. Stiffness of HIV-1 mimicking polymer nanoparticles modulates ganglioside-mediated cellular uptake and trafficking. Adv. Sci. 2020, 7, 2000649. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Sun, Q.; Ishii, T.; Kanehira, K.; Sato, T.; Taniguchi, A. Uniform TiO2 nanoparticles induce apoptosis in epithelial cell lines in a size-dependent manner. Biomater. Sci. 2017, 5, 1014–1021. [Google Scholar] [CrossRef]
- Yohan, D.; Cruje, C.; Lu, X.; Chithrani, D.B. Size-dependent gold nanoparticle interaction at nano–micro interface using both monolayer and multilayer (tissue-like) cell models. Nano-micro Lett. 2016, 8, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Wong, A.C.; Wright, D.W. Size-Dependent Cellular Uptake of DNA Functionalized Gold Nanoparticles. Small 2016, 12, 5592–5600. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Ma, S.; Nie, L.; Shang, X.; Hao, X.; Tang, Z.; Wang, H. Size-dependent endocytosis of single gold nanoparticles. Chem. Commun. 2011, 47, 8091–8093. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Au, L.; Zhang, Q.; Xia, Y. The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells. Small 2010, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Elbakry, A.; Wurster, E.C.; Zaky, A.; Liebl, R.; Schindler, E.; Bauer-Kreisel, P.; Blunk, T.; Rachel, R.; Goepferich, A.; Breunig, M. Layer-by-Layer Coated Gold Nanoparticles: Size-Dependent Delivery of DNA into Cells. Small 2012, 8, 3847–3856. [Google Scholar] [CrossRef]
- Simons, T.J. Passive transport and binding of lead by human red blood cells. J. Physiol. 1986, 378, 267–286. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.M.; Schürch, S.; Haenni, B.; Kapp, N.; Gehr, P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ. Sci. Technol. 2006, 40, 4353–4359. [Google Scholar] [CrossRef]
- Li, L.; Xi, W.-S.; Su, Q.; Li, Y.; Yan, G.-H.; Liu, Y.; Wang, H.; Cao, A. Unexpected Size Effect: The Interplay between Different-Sized Nanoparticles in Their Cellular Uptake. Small 2019, 15, 1901687. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, L.; Zhong, M.; Xiao, L.; Li, H.-W.; Wang, J. The morphology and surface charge-dependent cellular uptake efficiency of upconversion nanostructures revealed by single-particle optical microscopy. Chem. Sci. 2018, 9, 5260–5269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Candal, A.; Brown, T.; Krishnan, V.; Lopez-Loureiro, I.; Ávila-Gómez, P.; Pusuluri, A.; Pérez-Díaz, A.; Correa-Paz, C.; Hervella, P.; Castillo, J.; et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Control. Release 2019, 309, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Dizaj, S.M.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle–cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Zhang, K.; Fang, H.; Chen, Z.; Taylor, J.-S.A.; Wooley, K.L. Shape Effects of Nanoparticles Conjugated with Cell-Penetrating Peptides (HIV Tat PTD) on CHO Cell Uptake. Bioconjug. Chem. 2008, 19, 1880–1887. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J. Protein–Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles. Int. J. Nanomed. 2020, 15, 5783–5802. [Google Scholar] [CrossRef]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling evolution of protein corona: A prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef] [PubMed]
- Hadjidemetriou, M.; Kostarelos, K. Nanomedicine: Evolution of the nanoparticle corona. Nat. Nanotechnol. 2017, 12, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P.; Cho, N.-J. Kinetics of the formation of a protein corona around nanoparticles. Math. Biosci. 2016, 282, 82–90. [Google Scholar] [CrossRef]
- Van Hong Nguyen, B.-J.L. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [Green Version]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef]
- Hellstrand, E.; Lynch, I.; Andersson, A.; Drakenberg, T.; Dahlbäck, B.; Dawson, K.A.; Linse, S.; Cedervall, T. Complete high-density lipoproteins in nanoparticle corona. FEBS J. 2009, 276, 3372–3381. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; De Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: From nanostructure to uptake in cancer cells. Nanoscale 2014, 6, 2782–2792. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of surface charge on the colloidal stability and in vitro uptake of carboxymethyl dextran-coated iron oxide nanoparticles. J. Nanopart. Res. 2013, 15, 1874. [Google Scholar] [CrossRef] [Green Version]
- Bertoli, F.; Garry, D.; Monopoli, M.P.; Salvati, A.; Dawson, K.A. The Intracellular Destiny of the Protein Corona: A Study on its Cellular Internalization and Evolution. ACS Nano 2016, 10, 10471–10479. [Google Scholar] [CrossRef]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Müller, R.H. Functional groups on polystyrene model nanoparticles: Influence on protein adsorption. J. Biomed. Mater. Res. A. 2003, 65, 319–326. [Google Scholar] [CrossRef]
- Prozeller, D.; Pereira, J.; Simon, J.; Mailänder, V.; Morsbach, S.; Landfester, K. Prevention of Dominant IgG Adsorption on Nanocarriers in IgG-Enriched Blood Plasma by Clusterin Precoating. Adv. Sci. 2019, 6, 1802199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.R.; Gnopo, Y.D.; Gambinossi, F.; Mylon, S.E.; Ferri, J.K. Modulation of cell responses to Ag-(MeO2MA-co-OEGMA): Effects of nanoparticle surface hydrophobicity and serum proteins on cellular uptake and toxicity. J. Biomed. Mater. Res. A 2018, 106, 1061–1071. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Physicochemical properties of core–shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomedicine 2016, 12, 2149–2160. [Google Scholar] [CrossRef]
- Larson, T.A.; Joshi, P.P.; Sokolov, K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano 2012, 6, 9182–9190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyano, D.F.; Saha, K.; Prakash, G.; Yan, B.; Kong, H.; Yazdani, M.; Rotello, V.M. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano 2014, 8, 6748–6755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, X.; Gu, N. Computational investigation of interaction between nanoparticles and membranes: Hydrophobic/hydrophilic effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Bahrami, A.H.; Weikl, T.R.; Hall, C.K. Modeling nanoparticle wrapping or translocation in bilayer membranes. Nanoscale 2015, 7, 14505–14514. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, S.; Yan, X.; Zhou, H.; Zhan, J.; Liu, S.; Sharma, V.K.; Jiang, G.; Zhu, H.; Yan, B. Regulation of Cell Uptake and Cytotoxicity by Nanoparticle Core under the Controlled Shape, Size, and Surface Chemistries. ACS Nano 2019, 14, 289–302. [Google Scholar] [CrossRef]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal” Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Quattrocchi, N.; Van De Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.V. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Figueroa, S.M.; Fleischmann, D.; Beck, S.; Goepferich, A. The effect of ligand mobility on the cellular interaction of multivalent nanoparticles. Macromol. Biosci. 2020, 20, 1900427. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, J. Effects of ligand distribution on receptor-diffusion-mediated cellular uptake of nanoparticles. R. Soc. Open Sci. 2017, 4, 170063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubertová, V.; Martinez-Veracoechea, F.J.; Vácha, R. Influence of ligand distribution on uptake efficiency. Soft Matter 2015, 11, 2726–2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zhang, Y.; Chen, T.; Xiao, S.; Liang, H. Morphological and mechanical determinants of cellular uptake of deformable nanoparticles. Nanoscale 2018, 10, 11969–11979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhang, J.; Wang, Q.; Feng, D.; Liu, Q.; Yin, D.; Xu, Y.; Wei, B.; Ding, X.; Shi, X.; et al. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef]
- Wei, C.; Wei, R.; Jiang, G.; Jia, Y.; Lou, H.; Yang, Z.; Luo, D.; Huang, Q.; Xu, S.; Yang, X.; et al. Mechnical cues modulate ceullar uptake of nanoparticles in cancer via clathrin-mediated and caveolae-mediated endocytosis pathways. Nanomedicine 2019, 14, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajadi, M.S.; Atarod, M.; Sajjadi, M.; Isaabadi, Z. An Introduction to Green Nanotechnology, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2019; Volume 28, p. 356. [Google Scholar]
- Hui, Y.; Wibowo, D.; Liu, Y.; Ran, R.; Wang, H.-F.; Seth, A.; Middelberg, A.P.; Zhao, C.-X. Understanding the effects of nanocapsular mechanical property on passive and active tumor targeting. ACS Nano 2018, 12, 2846–2857. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Lu, H.; Yao, Y.; Ganda, S.; Stenzel, M.H. Length vs. stiffness: Which plays a dominant role in the cellular uptake of fructose-based rod-like micelles by breast cancer cells in 2D and 3D cell culture models? J. Mater. Chem. B 2018, 6, 4223–4231. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Zhang, M.; Kumar, S.; Vogus, D.R.; Menegatti, S.; Helgeson, M.E.; Mitragotri, S. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015, 9, 3169–3177. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Li, Y.; Li, X. Penetration of nanoparticles across a lipid bilayer: Effects of particle stiffness and surface hydrophobicity. Nanoscale 2019, 11, 4025–4034. [Google Scholar] [CrossRef]


| Physicochemical Property | Parameter | Nanoparticles | Cell Type | Uptake Mechanism | Important Features | Reference |
|---|---|---|---|---|---|---|
| Size | 5–100 nm | Ag | B16 | Clathrin-mediated endocytosis | Higher uptake of larger NPs; smaller cross plasma membrane faster | [45] |
| Shape | Nanospheres and nanostars | siRNA-conjugated-Au | U87 Glioblastoma | Endocytosis | Larger spheres (50 nm) and stars (40 nm) show higher uptake | [46] |
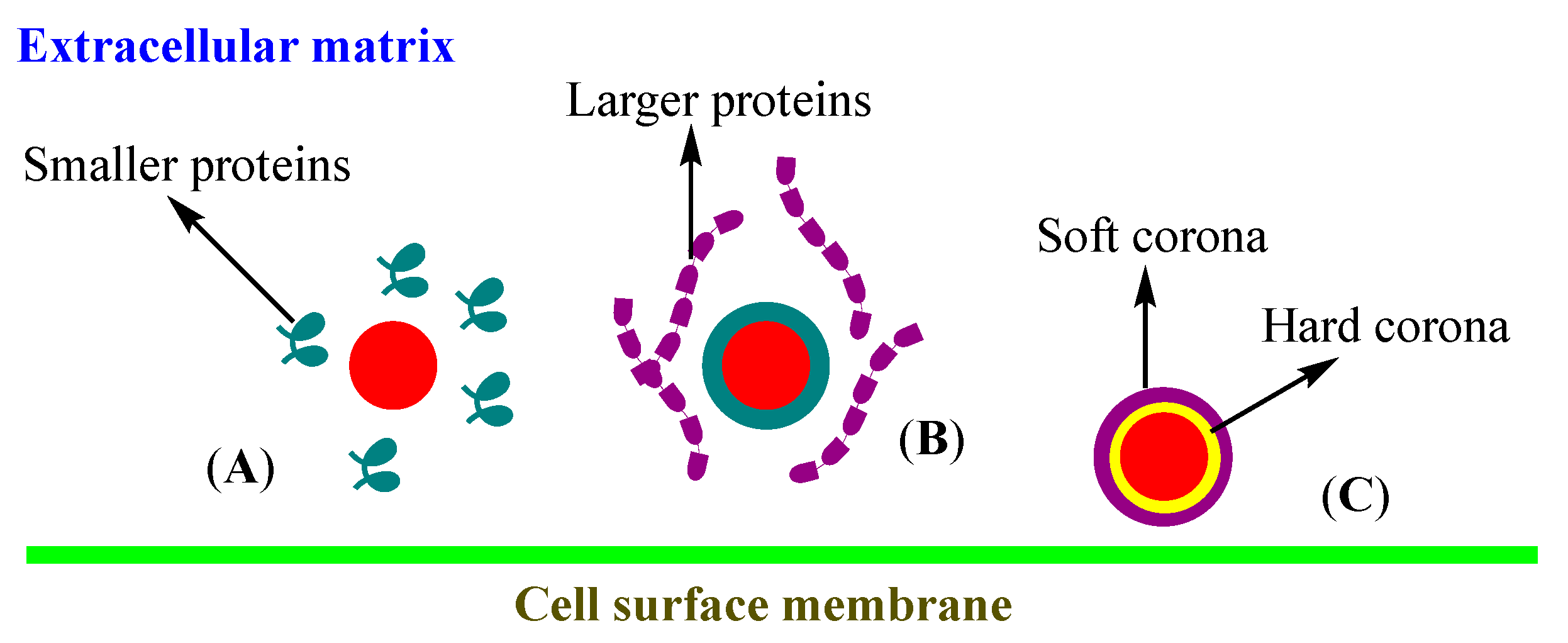
| Corona | Hard (HC) and soft (SC) corona proteins | Hard and soft corona modified silica and polystyrene | THP-1 and human brain endothelial | Endocytosis | Interactions of proteins on NP surface influence cell association | [47] |
| Surface charge | Positive, negative and neutral | PEG-b-PLA | Caco-2 and small intestinal epithelial | Clathrin and caveolin-mediated endocytosis | Positive charge improves uptake, transport, and distribution | [48] |
| Hydrophobicity | Nanogels | Amphiphilic polymeric system with varied hydrophibicity | Monocytic-like THP cells | Passive transport | Polymeric network hydrophobicity impacts protein binding and uptake | [49] |
| Ligand binding | Multivalent quantum dots 15–20 nm | Galactose-functionalized quantum dots | HepG2 | Caveolae- and clathrin-mediated endocytosis | Galactose multivalency, a key factor in uptake mechanism | [50] |
| Mechanical properties | Polymer stiffness | Ganglioside (GM3)-functionalized lipid-wrapped PLGA-PLA | CD169- expressing macrophages | Actin-dependent phagocytosis | Core stiffness influences NP uptake and localization | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. https://doi.org/10.3390/ijms21218019
Sabourian P, Yazdani G, Ashraf SS, Frounchi M, Mashayekhan S, Kiani S, Kakkar A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. International Journal of Molecular Sciences. 2020; 21(21):8019. https://doi.org/10.3390/ijms21218019
Chicago/Turabian StyleSabourian, Parinaz, Ghazaleh Yazdani, Seyed Sajad Ashraf, Masoud Frounchi, Shohreh Mashayekhan, Sahar Kiani, and Ashok Kakkar. 2020. "Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake" International Journal of Molecular Sciences 21, no. 21: 8019. https://doi.org/10.3390/ijms21218019